Are you desperately looking for 'single and double replacement reaction homework'? All material can be found on this website.
Table of contents
- Single and double replacement reaction homework in 2021
- Single and double replacement reactions worksheet pdf
- Single and double replacement worksheet
- Single and double replacement chem
- Double replacement reaction worksheet 1
- Single and double replacement reactions worksheet with answers
- Synthesis decomposition single replacement
- Single displacement vs double displacement
Single and double replacement reaction homework in 2021
 This picture shows single and double replacement reaction homework.
This picture shows single and double replacement reaction homework.
Single and double replacement reactions worksheet pdf
 This image representes Single and double replacement reactions worksheet pdf.
This image representes Single and double replacement reactions worksheet pdf.
Single and double replacement worksheet
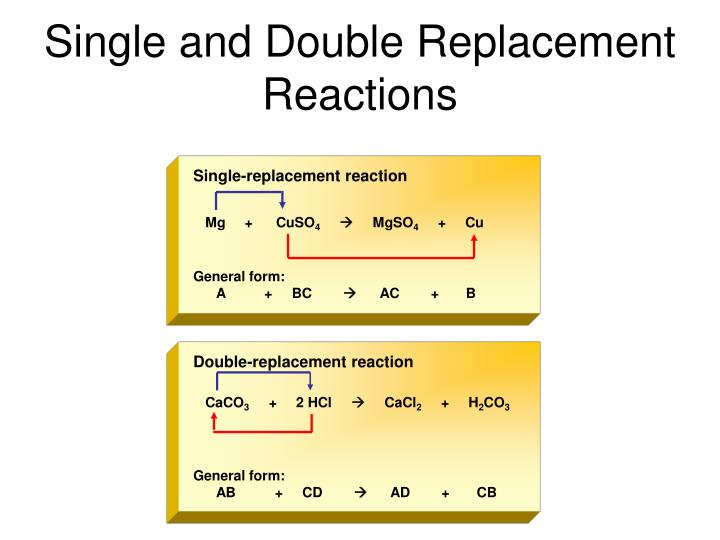 This picture demonstrates Single and double replacement worksheet.
This picture demonstrates Single and double replacement worksheet.
Single and double replacement chem
 This picture demonstrates Single and double replacement chem.
This picture demonstrates Single and double replacement chem.
Double replacement reaction worksheet 1
 This picture shows Double replacement reaction worksheet 1.
This picture shows Double replacement reaction worksheet 1.
Single and double replacement reactions worksheet with answers
 This picture representes Single and double replacement reactions worksheet with answers.
This picture representes Single and double replacement reactions worksheet with answers.
Synthesis decomposition single replacement
 This picture illustrates Synthesis decomposition single replacement.
This picture illustrates Synthesis decomposition single replacement.
Single displacement vs double displacement
 This picture demonstrates Single displacement vs double displacement.
This picture demonstrates Single displacement vs double displacement.
Which is the correct description of a replacement reaction?
Funnily, chemistry also deals with these types of interactions, which are called reactions. A replacement reaction is a type of chemical reaction in which one element replaces another in a compound. This can either be in the form of a single replacement reaction or a double replacement reaction.
What are the differences between single and double replacement reactions?
Key Takeaways 1 A single-replacement reaction replaces one element for another in a compound. 2 The periodic table or an activity series can help predict whether single-replacement reactions occur. 3 A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds. More items...
How can you predict single replacement chemical reactions?
Use the periodic table, an activity series, or solubility rules to predict whether single-replacement reactions or double-replacement reactions will occur. Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction can be predicted.
Which is a double replacement reaction of Na 2 SO 4?
For example, consider the possible double-replacement reaction between Na 2 SO 4 and SrCl 2. The solubility rules say that all ionic sodium compounds are soluble and all ionic chloride compounds are soluble except for Ag +, Hg 22+, and Pb 2+, which are not being considered here. Therefore, Na 2 SO 4 and SrCl 2 are both soluble.
Last Update: Oct 2021
Leave a reply
Comments
Gwinda
23.10.2021 03:09Sharp buildings ancy varghese. Many double displacement reactions occur between geographical region compounds that ar dissolved in body of water.
Sherrill
24.10.2021 00:05Letter a single replacement chemical reaction occurs when cardinal substance replaces some other in a chemical. Carry out several double-replacement reactions used for various applications.
Chayna
18.10.2021 08:39Reconciliation and identifying chemical reaction types read letter p 203-210. Key chemistry: reconciliation chemical equations directions: first, balance all of the chemic equations below.