Do you have a trouble to find 'write a balanced equation for combustion of octane'? Here you can find questions and answers on the topic.
The chemical equation for write a counterbalanced equation for burning of OctaneOctane is a hydrocarbon and an alkane with the chemical chemical formula C₈H₁₈, and the condensed structural chemical formula CH₆CH₃. Octane has many structural isomers that differ away the amount and location of branchy in the C chain. One of these isomers, 2,2,4-trimethylpentane is used equally one of the standard valu… this combustion reaction is: C8H18 + O2 --> CO2 + H2O. Apr 18, 2012 · The equation is special to the fire, but the universal form is: Atomic number 1 + Carbon + Oxygen = C Dioxide (in realised combustion) + Body of water.
Table of contents
- Write a balanced equation for combustion of octane in 2021
- Combustion of c8h18
- Combustion of gasoline equation
- How to balance combustion reactions
- What type of reaction is c8h18+o2=co2+h2o
- C8h18 combustion
- C8h18+o2=co2+h2o balanced equation
- Write a balanced chemical equation for the combustion of octane (c8h18)
Write a balanced equation for combustion of octane in 2021
 This picture shows write a balanced equation for combustion of octane.
This picture shows write a balanced equation for combustion of octane.
Combustion of c8h18
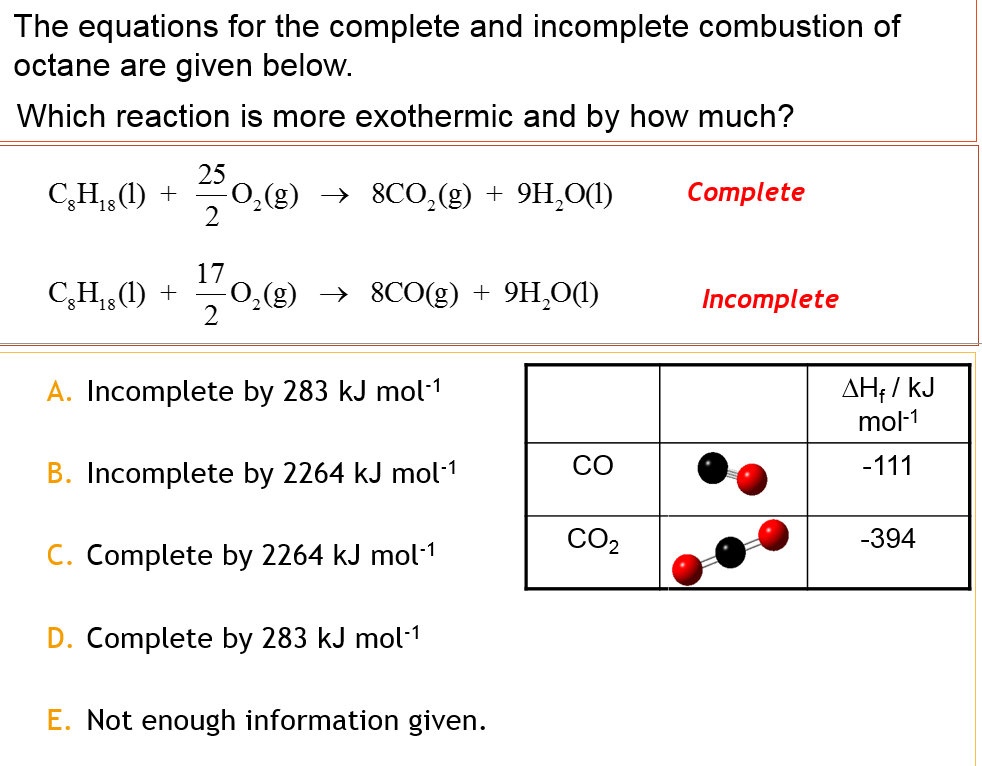 This picture illustrates Combustion of c8h18.
This picture illustrates Combustion of c8h18.
Combustion of gasoline equation
 This picture illustrates Combustion of gasoline equation.
This picture illustrates Combustion of gasoline equation.
How to balance combustion reactions
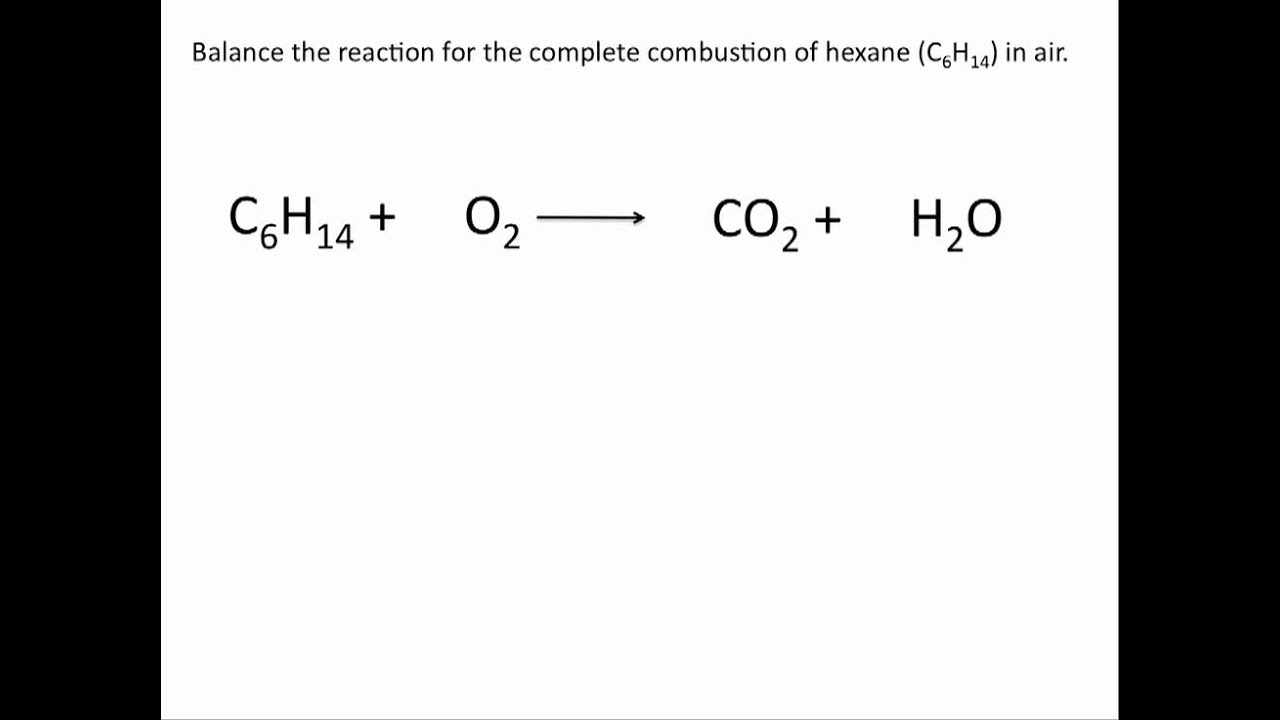 This image shows How to balance combustion reactions.
This image shows How to balance combustion reactions.
What type of reaction is c8h18+o2=co2+h2o
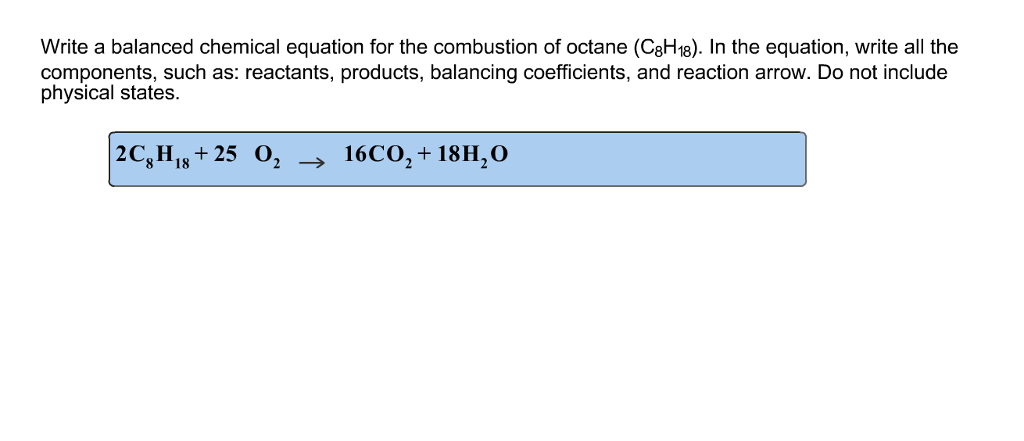 This picture shows What type of reaction is c8h18+o2=co2+h2o.
This picture shows What type of reaction is c8h18+o2=co2+h2o.
C8h18 combustion
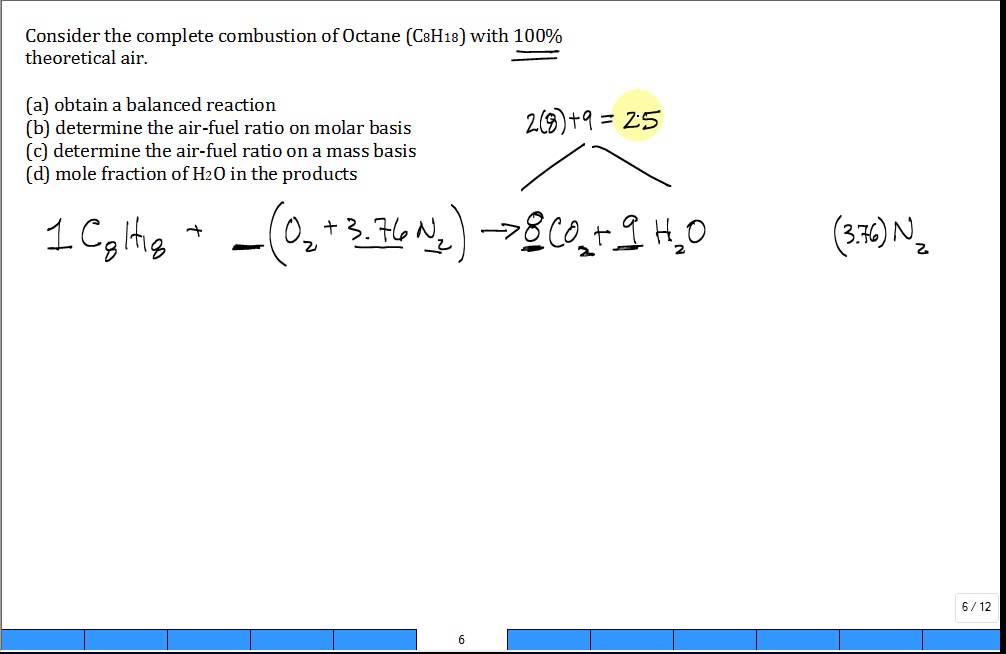 This picture illustrates C8h18 combustion.
This picture illustrates C8h18 combustion.
C8h18+o2=co2+h2o balanced equation
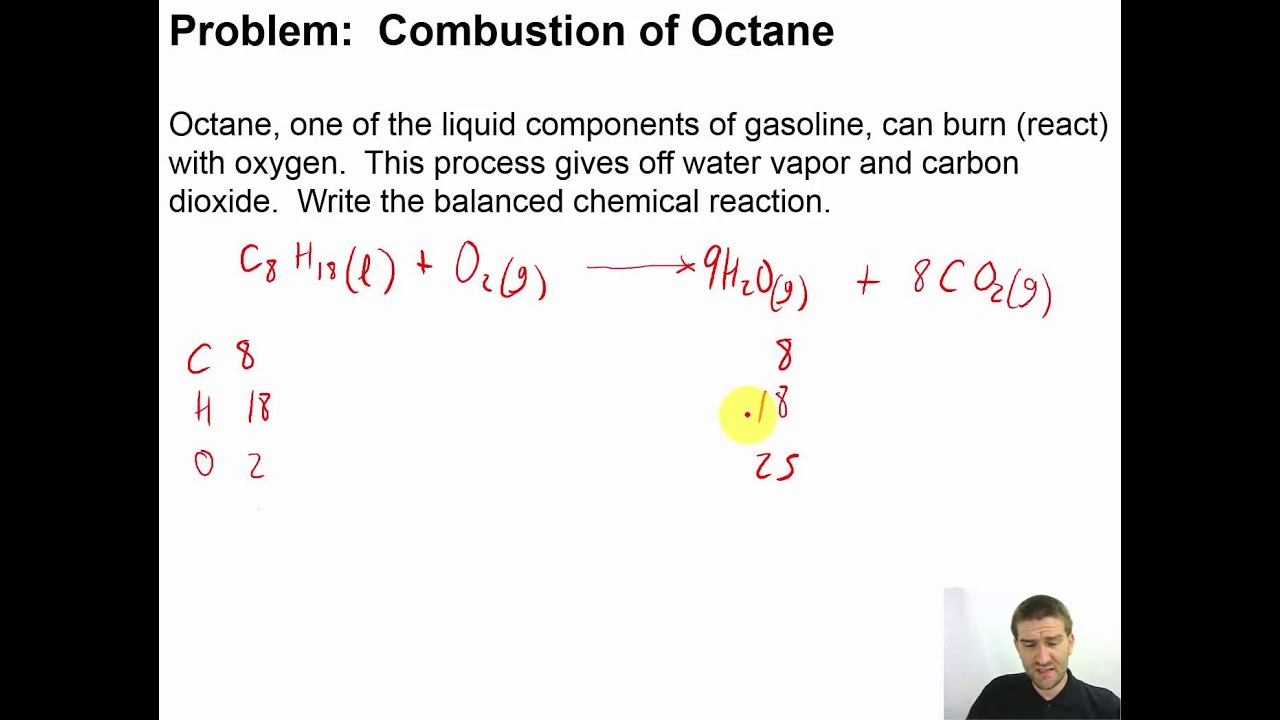 This picture illustrates C8h18+o2=co2+h2o balanced equation.
This picture illustrates C8h18+o2=co2+h2o balanced equation.
Write a balanced chemical equation for the combustion of octane (c8h18)
 This picture illustrates Write a balanced chemical equation for the combustion of octane (c8h18).
This picture illustrates Write a balanced chemical equation for the combustion of octane (c8h18).
What is the combustion reaction of octane produced by?
The combustion of a hydrocarbon produces carbon dioxide and water. Thus the combustion of octane is given by - To balance this equation, we need to balance the atoms on the left and right hand side. Octane has an excess of C and H and we use it as a reference. There are 8 carbon atoms and 18 hydrogen atoms in an octane molecule.
How do you balance the equation for the combustion of?
Since CO2 is a substance, you have to apply the coefficient to both C and two O atoms as they are all bonded to each other. Third, balance the next easiest atom. Now all that is left is to balance are the O atoms.
How many water vapours are in 2 octane gas?
The equation then becomes 2 octanes plus 25 diatomic oxygens yields 16 carbon dioxides and 18 waters, ie, 2 C8H18 + 25 O2 -> 16 CO2 + 18 H2O. 1 mole of octane gas + 12.5 moles of oxygen gas = 8 moles of carbon dioxide gas + 9 moles of water vapour + heat
How to find the chemical formula for Octane?
To obtain a equation containing whole numbers, we multiply the entire equation by 2. This gives the final equation. 2 C8H18 + 25 O2 ---> 16 CO2 +18 H2O. , studying chemistry this year. Remember : C8H18 is octane’s chemical formula.
Last Update: Oct 2021