Do you search for 'the aldol condensation synthesis of dibenzalacetone essay'? You can find questions and answers on the topic here.
Table of contents
- The aldol condensation synthesis of dibenzalacetone essay in 2021
- Dibenzalacetone mechanism
- The aldol condensation lab report
- Aldol
- Dibenzalacetone synthesis
- Theoretical yield of dibenzalacetone
- The aldol condensation synthesis of dibenzalacetone essay 07
- The aldol condensation synthesis of dibenzalacetone essay 08
The aldol condensation synthesis of dibenzalacetone essay in 2021
 This picture shows the aldol condensation synthesis of dibenzalacetone essay.
This picture shows the aldol condensation synthesis of dibenzalacetone essay.
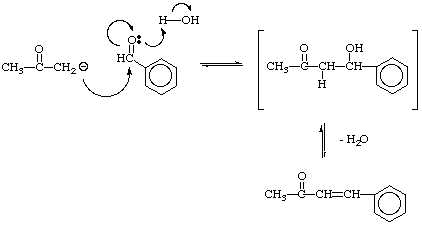
Dibenzalacetone mechanism
 This image illustrates Dibenzalacetone mechanism.
This image illustrates Dibenzalacetone mechanism.
The aldol condensation lab report
 This image shows The aldol condensation lab report.
This image shows The aldol condensation lab report.
Aldol
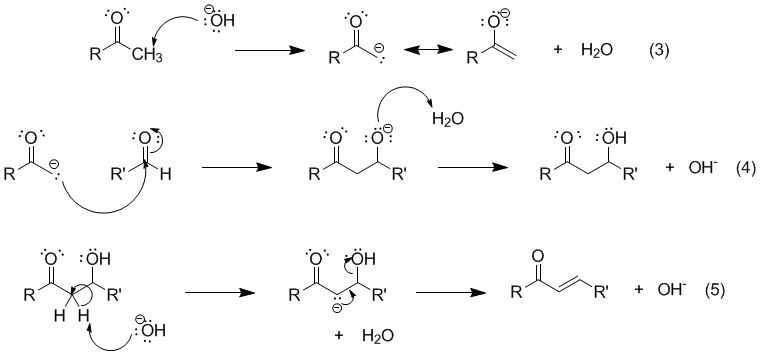 This image demonstrates Aldol.
This image demonstrates Aldol.
Dibenzalacetone synthesis
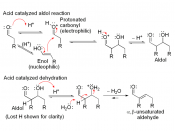 This picture illustrates Dibenzalacetone synthesis.
This picture illustrates Dibenzalacetone synthesis.
Theoretical yield of dibenzalacetone
 This picture illustrates Theoretical yield of dibenzalacetone.
This picture illustrates Theoretical yield of dibenzalacetone.
The aldol condensation synthesis of dibenzalacetone essay 07
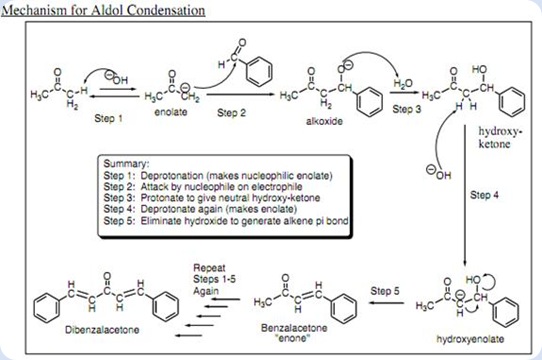 This picture representes The aldol condensation synthesis of dibenzalacetone essay 07.
This picture representes The aldol condensation synthesis of dibenzalacetone essay 07.
The aldol condensation synthesis of dibenzalacetone essay 08
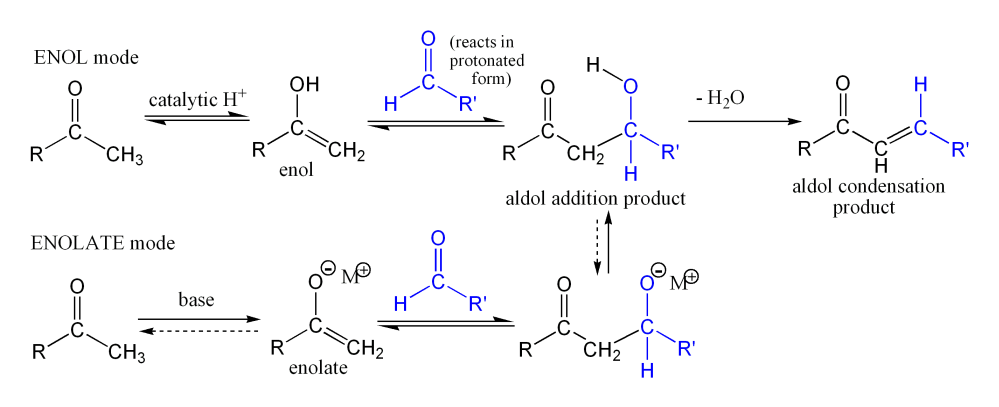 This image demonstrates The aldol condensation synthesis of dibenzalacetone essay 08.
This image demonstrates The aldol condensation synthesis of dibenzalacetone essay 08.
How is aldol condensation used to make dibenzalacetone?
Condensation is a process which joins two or more molecules usually with the loss of a small molecule such as water or an alcohol. Aldol condensation (Claisen-Schmidt reaction) definitely is a process which join two carbonyl groups with a loss of water molecule in order to form β-hydroxyketone.
Which is an example of an aldol condensation reaction?
The synthesis of dibenzalacetone is an illustration of a assorted Aldol condensation reaction. This experiment involves condensating propanone with two steps of Benz aldehyde ( giving dibenzalacetone. an organic Sun screen ) . The carbonyl group on the aldehyde is more reactive than that of the keytone.
Where can I find the aldol condensation lab manual?
The experimental procedure followed the format referenced in the lab manual Chemical Education Resources: Chem 236, Synt 720. The only experiment performed with the assistance of this lab manual was on page 101-103, Semi-Microscale Aldol Condensation.
How is the synthesis of dibenzalacetone formed?
IntroductionThe synthesis of dibenzalacetone is formed from an Aldol condensation reaction. An Aldol condensation reaction is a really effectual manner of organizing a C – C bond reaction. in which the enolate anion adds to the carbonyl group of the aldehyde.
Last Update: Oct 2021